QC and Me (A Guide to Agar Plating)
Hi everyone, today’s blog post is about the importance of a plating program within the brewery. Plating beer samples on a regular basis not only allows you to screen samples for potential yeast and bacterial contaminants that may spoil your product but also provides a visual insight to the health of your yeast, allowing you to establish consistent products. This blog post will cover the necessary equipment needed to implement and set up the quality control (QC) program, the various types of media available and their capabilities, as well as how to plate various samples including yeast slurry and beer samples. As mentioned in previous blog posts and in my company bio, I am a big fan of pugs so let’s keep this blog post-on-brand and get started.

Pugs doing science is just amazing. And people say they aren’t smart.
I bet he is developing a diastatic-free Saison yeast.
Implementing a QC Plating Program
Setting up and purchasing the necessary equipment for pouring agar plates can be an expensive upfront cost. But once all the equipment is purchased, making the media, pouring plates, and keeping the QC program running is fairly cost-effective. Routine screening of your product will also give you more confidence in your product and provide you with records of quality control monitoring, should issues arise later down the road. Additionally, testing samples through various points throughout the brewing process (ex. when beer is in the BT, FV, and after packaging) will allow you to pinpoint if and when the product may have been exposed to contaminants so improved preventative measures can be taken during those critical control points.
In order to prepare your own media in-house, you will need an autoclave, a stir plate, a magnetic stir bar, some autoclave tape, sterile petri dishes, Bunsen burner, and pyrex glass bottles/flasks. The autoclave is easily the most expensive piece of equipment, however, it can be used to sanitize other brewing equipment including gaskets, clamps, and media bottles that are rated for autoclave use (made of polypropylene or glass) which can help justify the cost as it won’t have only one purpose. The stir plate and stir bar are needed to homogenize the media after autoclaving to ensure it is evenly mixed before pouring plates. All of the other listed equipment is needed to ensure aseptic techniques are maintained throughout the plate pouring process. When pouring the plates, fill the sterile petri dishes half-way aseptically by working closely to the flame from the Bunsen burner. Let them sit for 2 days at room temp before using them, and then bag them and place them in a fridge for long term storage (~2 month shelf life).

A properly filled agar plate, where the media is filled half-way and stops just under the lid line.
Now, this may sound like a lot of work and you are absolutely correct – it is. If you would rather purchase plates to use internally, Escarpment Labs can provide brewers with agar plates, and offers quality control services for a relatively low cost if you want to avoid all of the hassle of setting up your own quality control program. To find out more about these services, feel free to reach out.

This is how happy you will be when your plates show up already prepared
and you spend your free time looking at pug gifs instead.
All that is left to do is to choose the media that best fits your needs and begin sampling/plating samples on a routine basis.
Selecting Media for your QC Program
Once you have all the necessary equipment to begin a QC program, you will need to decide what media you want to plate your samples on – and this decision comes down to what is important for you to screen for based on the needs and bugs used in your brewery. Are you looking to screen for diastatic yeast or for a wild yeast contamination? Lactic Acid Bacteria (LAB) contaminations or to make sure your Lacto pitch can be re-used? Maybe you are using a clean ale yeast and a saison yeast, and want to ensure that there is no cross-contamination between the two after cleaning the tank and pitching the next strain into the same vessel?
Fortunately, there is a wide variety of selective and/or differential agar media available to test for all of this – but choosing the best combination for your brewery is important to ensure you are not wasting time, money, or effort. Selective media use some kind of selective agent to restrict the growth of non-target organisms. For example, LCSM agar uses copper sulfate to restrict the growth of many brewing yeasts and allow for wild yeast contaminations to be revealed. Differential media use some kind of property to differentiate microbial colonies from each other. For example, WLN/WLD contain bromocresol green, which turns yellow when the pH is dropped by acid forming bacteria.
The following table describes the most popular media used in the industry, what they screen for, and what they can’t do. A good QC program will include a several different media to screen for a range of issues, but not too many to screen for everything that may not be relevant. For example, media to screen for domesticated yeast, wild yeast, and lactic acid-producing bacteria (LABs) is a great starting point until you learn the needs of your brewery.
| Media | Purpose: | Limitations |
|---|---|---|
| WLN (Nutrient Agar) |
A differential medium to screen for different yeast colony morphology. This will let you see if there is yeast cross-contamination. | Will not tell you if the contamination is wild or domestic. Also allows growth of bacteria. |
| WLD (must add/contain cycloheximide) |
This medium selects for only cycloheximide-resistant wild yeast | Only see growth if yeast is cycloheximide resistant, so not all wild yeast. Also allows growth of cycloheximide-resistant aerobic bacteria |
| MRS (must add/contain cycloheximide) |
Selective medium to screen for bacteria (can be used aerobically or anaerobically to differentiate) | Inhibits growth of yeast, tailored to growth of lactic acid bacteria – other species may not grow as well. |
| LCSM | Selective medium to screen for copper-resistant, non-sacch wild yeast. Can also be used to detect Saccharomyces diastatic yeast*) | Only copper-sulfate resistant yeast grows, so not all wild yeast can be detected. Some Saccharomyces strains can grow including diastaticus. |
| LMDA (cycloheximide optional) |
This medium screens for both brewer’s yeast and lactic acid bacteria, encouraging growth of many microorganisms. | Contains some buffering salts which tend to precipitate unevenly if poured by hand – must be mixed and poured very carefully and quickly. Automated plate pourers are recommended. |
| LWYM (must add crystal violet) |
Uses crystal violet and fuchsin to restrict growth of brewing yeast and select for wild yeasts. | Need to verify against your house strain, as some domesticated Saccharomyces strains grow on LWYM. Does not differentiate for non-Saccharomyces yeast |
| Lysine | This medium screens for wild yeast that can utilize and digest lysine as a nitrogen source | Some brewing yeasts can grow on this medium. |
| UBA (can add cycloheximide to make selective) |
General purpose medium containing beer which supports growth of many different brewing microbes. | Hard to determine type of contamination (ex. wild yeast vs. in-house cross contamination). Beer used in medium may introduce some inconsistency. |
| MacConkey Agar | This medium screens for enteric bacteria, suitable for screening water and wort streams for contamination. | Can not be used to screen for yeast or LABs. |
| HLP (tubes, not plates) | Semi solid agar medium in tubes. Lactic acid bacteria diffuse in the agar, forming teardrop shapes. Can allow for low oxygen incubation without an anaerobic incubator. | Challenging to perform analysis on positive samples. Colonies on plates are more amenable to secondary identification tests. |
*A good indicator of the presence of diastatic yeast, however, not all diastatic yeast grow on LCSM and some non-diastatic yeast are capable of growing as well.
There are many more media available in addition to the ones listed above, and to read about them as well as the ones mentioned in more detail follow this link to the MBAA’s website.
Plating Samples onto Media Agar Plates
Once you have your agar plates and have samples to test (that have been taken aseptically from a brite tank or fermenting vessel), you are ready to begin plating. To do this, you will need a dedicated area to plate, where you have access to a Bunsen burner and are able to sanitize the surface using 70% isopropanol. There are 2 ways to plate a sample, and the method you choose is dependent on the type of sample.
1. Spread Plate (thin slurries and beer)
This method is used when you are plating thin or diluted samples that may contain low levels of contaminations and should not be used for yeast slurries or re-hydrated yeast. This process involves plating 500µL (0.5mL) onto a plate using a pipette, and then moving the sample around the plate with a glass rod. Samples requiring greater sensitivity can be aseptically filtered through a 0.22 or 0.45µM filter, and the filter (catching any possible microbial cells) can be incubated on agar. This process is a lot to explain, so I created this video to show you the step by step instructions.
Spread Plate Step by Step Instructions:
- Flame opening of the sample vessel.
- Take 500 µL (0.5 mL) and inject onto a plate, making sure not to touch the plate with the tip.
- Immerse glass spreader in 95% ethanol for at least one minute, then burn off the ethanol.
- Tap glass rod onto an area of the agar plate without a sample to cool down, then spread sample evenly over the agar.
- Incubate plates.

2. Streak Plate (Thick yeast slurries, yeast differentiation on WLN)
Streak plates are the more common method for thick yeast slurries, as it uses much less sample (10µL) and allows you to separate individual colonies on the agar for more qualitative analysis. To perform this, all you will need is an inoculation loop. Again, the following video shows step by step instructions.
Streak Plate Step by Step Instructions:
- Flame loop, wait until it glows bright orange and wait at least 3 seconds.
- Flame sample opening.
- Place loop into the sample, move it around until it cools. Pull loop straight up out of the sample and make sure the loop has liquid in it.
- Streak sample onto 1/3 of the plate.
- Re-flame the loop the same as in step 1, the touch down on the agar where you haven’t streaked yet.
- Streak into the original streak 3X, then continue to streak the rest of the plate without crossing into any of the original streaks to isolate individual colonies.
- Incubate plates.
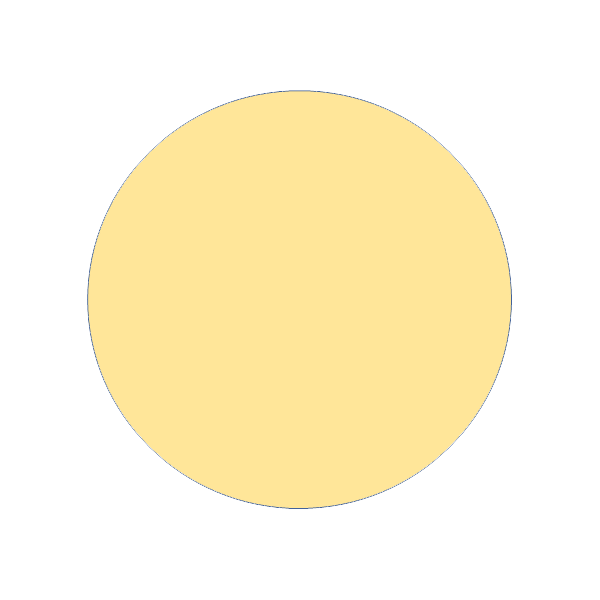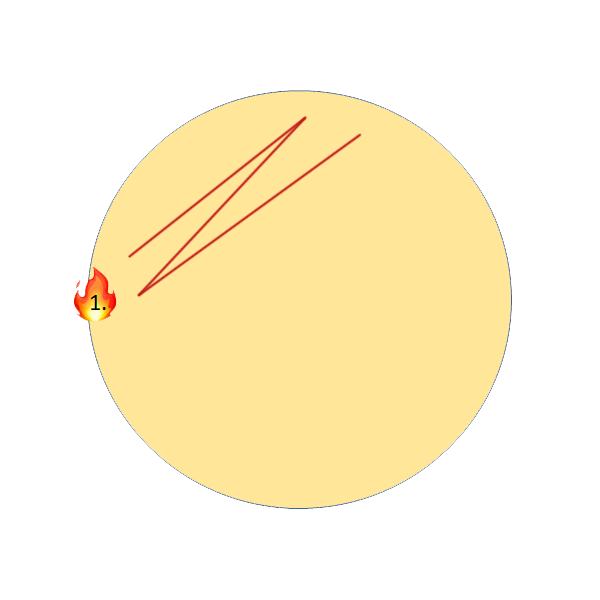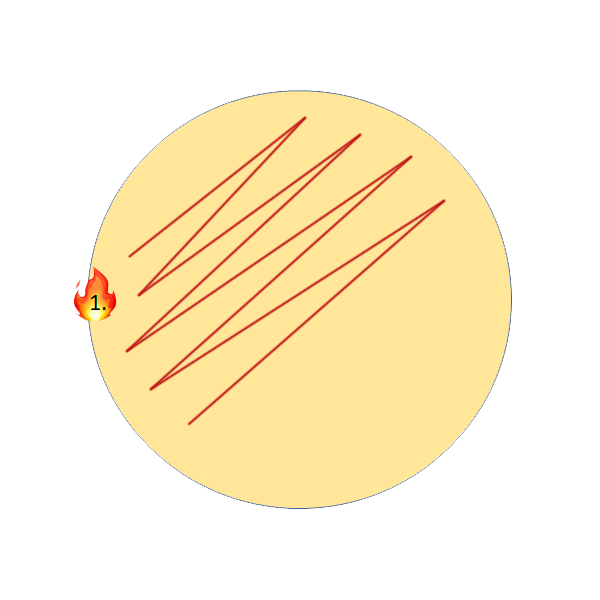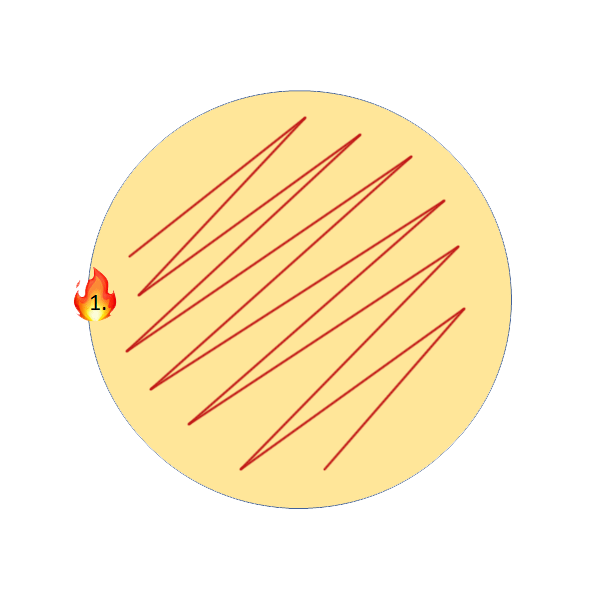

I personally recommend one of these two patterns, with a tendency to favour the second more as it allows for better isolation of single colonies, should they exist in the sample. This is also the pattern I performed in the above video demonstration.
Once the samples have been streaked onto the agar plates, they will need to be incubated. For most samples, aerobic incubation at 25°C is ideal, however, some plates such as MRS where you are screening for anaerobic growth will need to be incubated in an oxygen-free environment. This can be as simple as a heavily purged Cornelius keg or as sophisticated as a specially designed anaerobic incubator.
That is all there is to it, and now you know the basics of plating. Adding routine plating to your existing QC program will add immense value, as it will allow you to better understand what is happening to your product at multiple stages of the brewing process. This allows you to make decisions as to when to replace your yeast if it becomes contaminated, how to better clean filling equipment if you are seeing contamination post-packaging but not in the fermenter, and provides you with a paper trail of due diligence (I recommend saving pictures of your plates and writing up a report for each batch of beer produced for traceability). If you have any questions, feel free to reach out by commenting below or contacting us at qc@escarpmentlabs.com.
Now, get streaking!

1 comment
Hey! I have a few questions. I would really appreciate it, if I could get an answer.
What aliquot do you suggest for spread plating on 60×15mm petri dishes?
Which agar do you suggest for analysis of bright beers?
Lastly, do you suggest spread plating or membrane filtration for bright beer analysis?
Thanks!


